3
Figure 3
. Forward and reverse primers for amplifying Citrine-YFP/CFP tags to use in TT-PCR. Orange
boxes indicate the FseI and SfiI sites in the forward and reverse primers, respectively. Green boxes
indicate the (Gly)
5
Ala and AlaGly(Ala)
5
GlyAla linkers in the forward and reverse primers, respectively.
The N-terminal sequence of Citrine-YFP/CFP contained in the forward primer is indicated in blue.
FseI
SfiI
AlaGly(Ala)
5
GlyAla
(Gly)
5
Ala
Citrine/CFP N-terminus
forward primer citrine/CFP
5’-GGC CGG CCT GGA GGT GGA GGT GGA GCT
GTG AGC A
-3’
G R P
G G G G G A V S
reverse primer citrine/CFP
5’-GGC CCC AGC GGC CGC AGC AGC ACC AGC AGG ATC-3
’
A G A
A A A A G A P D
The PCR products are gel-purified using the GFX PCR purification kit (Amersham) or PCR
Purification Kit (Qiagen) to remove dNTPs, primers and enzyme, and used in TT-PCR (see below).
II. Gene tagging
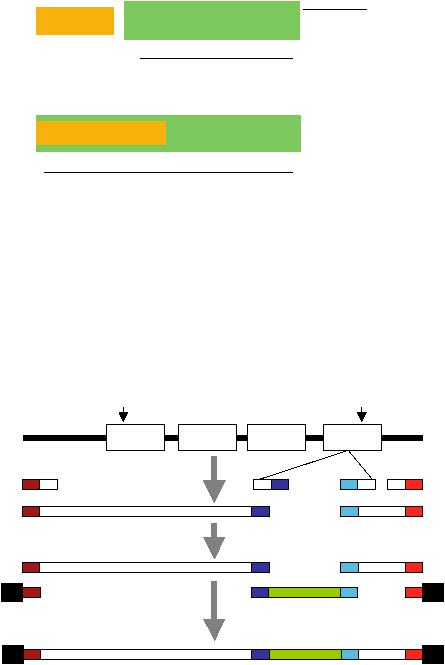
The entire protocol is summarized in Figure 4 and described in detail below.
Figure 4
. Flowchart for the gene tagging protocol. White boxes represent gene-specific sequences, dark and lig
red boxes represent P1 and P2 primer sequences overlapping the forward attB1 and reverse attB2 Gateway
primers, respectively, and dark and light blue boxes represent P2 and P3 primer sequences overlapping the
fluorescent tag primers (see Figure 5).
exon 1
exon 2
exon 3
exon 4
+1 kb
3’ UTR
-3 kb
5’ UTR
target
gene
P1
P2
P3
P4
1st
PCR
2nd
PCR
(TT-PCR)
Citrine-YFP
or
CFP
forward attB1
Gateway
primer
reverse attB2
Gateway
primer
fluorescently-tagged full-length gene
ATG
STOP
1. First PCR reaction
a. Genomic DNA template
Genomic DNA is extracted from leaf material of 6-week-old A. thaliana ecotype Columbia
plants using the DNeasyÒ Plant Mini Kit (Qiagen) according the manufacturer’s instructions.

